

21 Kansai Medical University Hospital, Osaka, Japan.20 Chris O'Brien Lifehouse, Camperdown, New South Wales, Australia.19 Hospital Universitario Quirónsalud Madrid, Madrid, Spain.18 Department of Oncology, University of Turin, Azienda Ospedaliero-Universitaria San Luigi, Orbassano, Italy.17 David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA.
16 LungenClinic, Airway Research Center North, German Center for Lung Research, Grosshansdorf, Germany.15 Epworth Healthcare, Richmond, Victoria, Australia.14 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy.13 Soroka Medical Center, Ben-Gurion University, Beer Sheva, Israel.11 Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.9 Southern Medical Day Care Centre, Wollongong, New South Wales, Australia.8 Department of Respiratory and Critical Care Medicine, Karl Landsteiner Institute of Lung Research and Pulmonary Oncology, Vienna, Austria.7 Westmead Hospital and University of Sydney, Sydney, New South Wales, Australia.6 Hospital Universitario Fundación Jiménez Díaz, IIS-FJD, Madrid, Spain.5 Vall d'Hebron University, Vall d'Hebron Institute of Oncology, Barcelona, Spain.


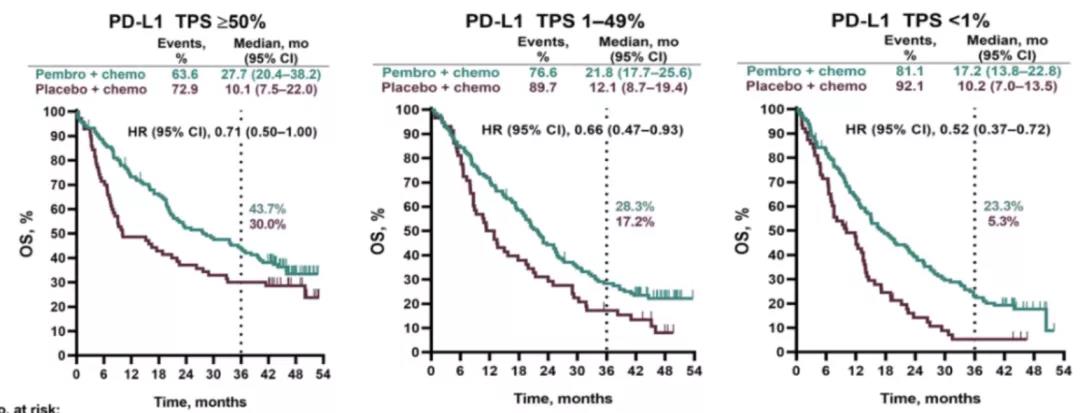
At data cutoff, 79 pts in each group had begun subsequent therapy 4 pts began a second course of pembro.Ĭonclusion: Similar to the global KEYNOTE-042 study, first-line pembro continues to prolong OS and provide durable response in pts in China with advanced/metastatic PD-L1-positive NSCLC without EGFR/ALK alterations after nearly 4 y of follow-up. Among 22 pts who completed 35 cycles of pembro, ORR was 81.8% (95% CI, 59.7%-94.8%) estimated OS rate 4 y after randomization was 69.1%. 19.5% and 68.8% of pts in the pembro and chemo groups experienced treatment-related grade 3-5 AEs. Median time from randomization to data cutoff (Apr 28, 2021) was 47.2 (range, 39.8-56.1) mo. Results: 262 pts with PD-L1 TPS ≥1% were randomized in China to pembro (n = 128) or chemo (n = 134). No alpha was allocated to the China extension analysis. Eligible pts who completed 35 cycles of pembro could receive a second course of pembro. Primary endpoints were OS in pts with PD-L1 TPS ≥50%, ≥20%, and ≥1%. Methods: Pts enrolled in China in the KEYNOTE-042 global (NCT02220894) and China extension (NCT03850444) studies were randomized 1:1 to pembro 200 mg Q3W for ≤35 cycles or carboplatin+paclitaxel or pemetrexed with optional pemetrexed maintenance (nonsquamous only). We present efficacy and safety outcomes with an additional 14 calendar mo of follow-up in Chinese pts in KEYNOTE-042. Background: In the global, phase 3 KEYNOTE-042 study, pembrolizumab (pembro) significantly prolonged OS vs chemotherapy (chemo) in patients (pts) with previously untreated advanced/metastatic NSCLC with PD-L1 TPS ≥1% without EGFR/ALK alterations.


 0 kommentar(er)
0 kommentar(er)
