
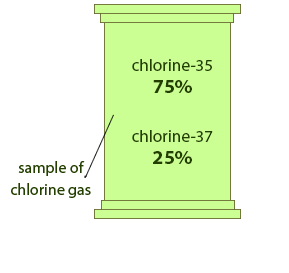
Both these isotopic forms give the apparent atomic weight of chlorine which is equal to 35.453 g/mol. The chlorine 37 isotope tends to account for about 24.23% of the natural chlorine content, while the other stable isotope of chlorine, chlorine 35, accounts for about 75.77% of total chlorine content. The sum of 17 protons and 20 neutrons in this atomic nucleus makes a total of 37 nucleons. We can write the symbol of this isotope as 37Cl.

It is one of the stable isotopes of chlorine chemical element. What is Chorine 37?Ĭhlorine 37 is an isotope of chlorine chemical element, and it has 17 protons and 20 neutrons in its atomic nuclei. Additionally, there are some extremely rare forms of chlorine isotopes having a half-life below 1 hour. In addition, there is a radioactive isotope of chlorine (chlorine 36) that occurs in trace amounts in nature. The abundance of this isotope in nature is about 75.77%.Ĭhlorine 35 and chlorine 37 contribute to the calculation of the standard atomic weight of chlorine chemical element, which is 35.45. It is the most stable and abundant isotope of chlorine.
Side by Side Comparison – Chlorine 35 vs 37 in Tabular FormĬhlorine 35 is an isotope of chlorine chemical element, and it has 17 protons and 18 neutrons in its atomic nuclei. Similarities Between Chlorine 35 and 37ĥ. These three forms differ from each other according to the number of neutrons per atomic nuclei. There are three major isotopes of chlorine, which are named chlorine-35, chlorine-36 and chlorine 37. Section 11, Table of the Isotopes.The key difference between chlorine 35 and 37 is that chlorine 35 has 18 neutrons per atomic nuclei, whereas chlorine 37 has 20 neutrons per atomic nuclei.Ĭhlorine is a chemical element having the atomic number 17 and chemical symbol Cl. Holden in CRC Handbook of Chemistry and Physics, 85th Edition, online version. Information extracted from the NuDat 2.1 database (retrieved Sept. National Nuclear Data Center, Brookhaven National Laboratory.The Nubase2003 evaluation of nuclear and decay properties, Nuc. Editing notes on this article's talk page. Half-life, spin, and isomer data selected from these sources.Isotopic compositions and standard atomic masses from Atomic weights of the elements.Bersillon in Nuclear Physics A729 (2003). Isotope masses from Ame2003 Atomic Mass Evaluation by G.Uncertainty values denote one standard deviation, except isotopic composition and standard atomic mass from IUPAC which use expanded uncertainties. Uncertainties are given in concise form in parentheses after the corresponding last digits.Spins with weak assignment arguments are enclosed in parentheses. Values marked # are not purely derived from experimental data, but at least partly from systematic trends.Substantial deviations from the given mass and composition can occur. Commercially available materials may have been subjected to an undisclosed or inadvertent isotopic fractionation.The uncertainty in the atomic mass may exceed the stated value for such specimens. Geologically exceptional samples are known in which the isotopic composition lies outside the reported range.36Cl has seen use in other areas of the geological sciences, including dating ice and sediments. Thus, as an event marker of 1950s water in soil and ground water, 36Cl is also useful for dating waters less than 50 years before the present. The residence time of 36Cl in the atmosphere is about 1 week. Additionally, large amounts of 36Cl were produced by irradiation of seawater during atmospheric detonations of nuclear weapons between 19. The half-life of this hydrophilic nonreactive isotope makes it suitable for geologic dating in the range of 60,000 to 1 million years. 36Cl decays to 36 S and to 36 Ar, with a combined half-life of 308,000 years. In the subsurface environment, 36Cl is generated primarily as a result of neutron capture by 35Cl or muon capture by 40 Ca.
36Cl is produced in the atmosphere by spallation of 36 Ar by interactions with cosmic ray protons. Trace amounts of radioactive 36Cl exist in the environment, in a ratio of about 7x10 −13 to 1 with stable isotopes.


 0 kommentar(er)
0 kommentar(er)
